FDA announces new Class I recall of CPET devices due to choking hazard
The U.S. Food and Drug Administration (FDA) announced that Vyaire Medical has recalled the twin tube samples lines from its Vyntus CPX Metabolic Cart due to ongoing safety concerns. This is a Class I recall, which means the FDA believes using these devices “may cause serious injuries or death.”
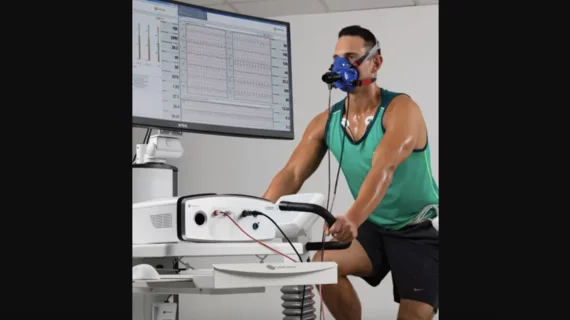
The Vyntus CPX is a cardiopulmonary exercise test (CPET) system designed to evaluate gas samples from a patient’s breath while they use a treadmill or bicycle ergometer. It was designed to collect “breath-by-breath data” from both adult and pediatric patients and can perform standalone 12-lead electrocardiograms if used in tandem with additional equipment.
This recall is focused on ongoing issues with the Vyntus CPX system’s twin tubes, which help evaluate the patient’s breath by assessing oxygen and carbon dioxide levels. The tubes have been linked to a heightened risk of separation.
“The separated component may fall into the patient’s mouth resulting in a potential choking hazard which may lead to an airway obstruction, requiring medical intervention to prevent further injury or harm,” according to an advisory shared by the FDA.
No injuries or deaths have been associated with this recall.
Vyaire Medical alerted customers about this issue back in April, sending an Urgent Field Safety Notice that asked them to isolate any devices that may be affected. The alert also included instructions on how to perform a test on the devices to verify their twin tube connections.
Click here for more details from the FDA about this Class I recall.