Medtronic heart rhythm technologies on full display at AF Symposium 2025
Medtronic’s heart rhythm management portfolio, including its insertable cardiac monitors (ICMs) and pulsed field ablation (PFA) systems, have had a significant footprint at AF Symposium 2025, a three-day conference in Boston focused on advanced atrial fibrillation (AFib) technologies.
Insertable cardiac monitors deliver AI-guided risk assessments
First, researchers presented initial results from the DEFINE AFib trial, which found that Medtronic’s Linq family of ICMs were able to work together with advanced artificial intelligence algorithms to guide treatment decisions for AFib patients. Nearly 1,000 patients were evaluated and then organized based on their risk of requiring AFib-related healthcare. Overall, 22% of trial participants who landed in the high-risk category did require care as predicted within less than five months.
“The first-of-its-kind DEFINE AFib study leveraged a unique design that engaged patients from the very beginning,” Jonathan P. Piccini, MD, chair of the DEFINE AFib clinical study steering committee and an electrophysiologist with Duke University Hospital and the Duke Clinical Research Institute, said in a statement. “We know that how much AFib a patient experiences matters, but we don’t know how different durations or patterns impact the risk of future health events. Combining continuous rhythm monitoring with traditional risk factors has helped clarify how AFib burden and patterns can inform risk, prioritization, and treatment decisions.”
A separate subanalysis of DEFINE AFib presented at European Society of Cardiology (ESC) Congress 2024 associated the Linq ICMs with certain treatment benefits over the Apple Watch. For instance, 40% of AFib episodes were found to take place when patients were not even wearing their Apple Watch. Also, even when worn, the Apple Watch detected just 26% of AFib episodes spotted by the Linq devices.
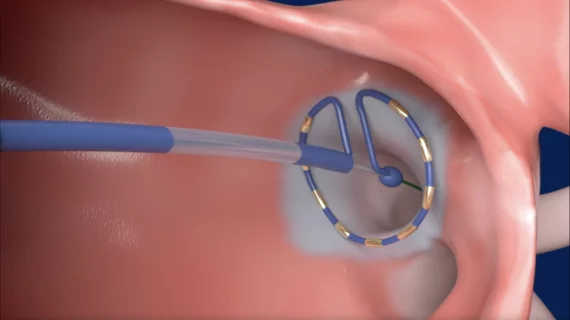
New pulsed field ablation data presented in Boston
Medtronic’s two PFA offerings, the Affera Mapping and Ablation System with the Sphere-9 catheter and the PulseSelect System, are featured in several presentations during the AF Symposium.
The PulseSelect PFA System was the very first PFA offering to gain U.S. Food and Drug Administration (FDA) approval when the agency cleared its use for both paroxysmal and persistent AFib in December 2023. The Affera Mapping and Ablation System with the Sphere-9 catheter followed suit when it gained approval in October 2024.
Sessions at AF Symposium are scheduled to focus on how these technologies can assist cardiologists with pulmonary vein isolation procedures, the likelihood of same-day discharge for PFA patients and the Affera system’s impact on fluoroscopy usage. Another five case presentations are on the schedule as well, giving conference attendees a close look at PFA’s potential.
“These new data and cases at AFib Symposium will provide physicians with additional valuable insight as awareness and use of our PFA offerings continue to increase around the world,” Rebecca Seidel, president of Medtronic’s cardiac ablation solutions business, said in a statement.