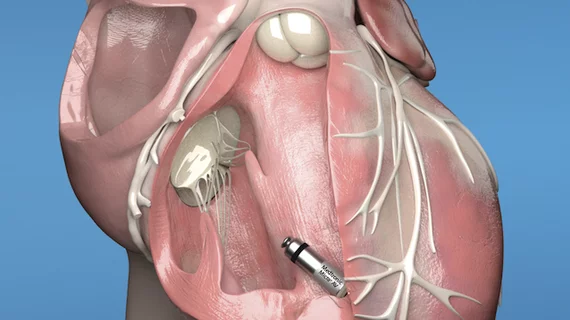
Medtronic receives CE mark approval for next-generation leadless pacemakers
Medtronic has gained European CE mark approval for its next-generation Micra leadless pacemakers, the Micra AV2 and Micra VR2 transcatheter pacing systems. Both devices gained U.S. Food and Drug Administration approval in May 2023.
The original Micra leadless pacemaker gained significant attention due to its small size—Medtronic regularly calls it “the world’s smallest pacemaker”—and the fact that it is implanted inside a patient’s heart rather than under the armpit. These updated models were designed with a much longer battery life. The Micra AV2 battery is projected to last 16 years, and the Micra VR2 battery is projected to last 17 years. In addition, updated artificial intelligence algorithms help the Micra AC2 device automatically program AV synchrony.
No chest incision is required for patients to receive either device; an interventional cardiologist is able to implant them via a minimally invasive procedure.
“For more than eight years, our Micra leadless pacemakers have provided meaningful benefits to people in Europe who require a pacemaker,” Robert C. Kowal, MD, PhD, general manager of cardiac pacing therapies within Medtronic’s cardiac rhythm management business, said in a statement. “Now, these patients have access to the latest leadless pacing technology that, for most of them, may be the only device they will ever need.”
Medtronic estimates more than 200,000 patients have already received a Micra pacemaker. The company also reviewed its long history in the cardiac pacing space, noting that it is credited with inventing the wearable pacemaker in 1957 and then introducing the first MRI-compatible pacemakers to the market in 2011. The first leadless pacemakers followed in 2015.
The CE mark confirms a product has met all applicable health, safety and environmental requirements put forth by the EU. The CE mark is required for products to be marketed and sold in dozens of countries, including Germany, Spain, Italy and the United Kingdom.