Real-world patients now being treated with Medtronic’s PFA system
Medtronic announced Thursday, Feb. 1, that commercial patients around the world are now being treated with its PulseSelect pulsed field ablation (PFA) system. Back in December, PulseSelect was the first PFA system approved by the U.S. Food and Drug Administration (FDA) for the treatment of atrial fibrillation (AFib).
Less than two months later, Boston Scientific’s Farapulse became the second PFA system to receive FDA approval. However, while PulseSelect is approved for both paroxysmal and persistent AFib, Farapulse is only approved to treat paroxysmal AFib.
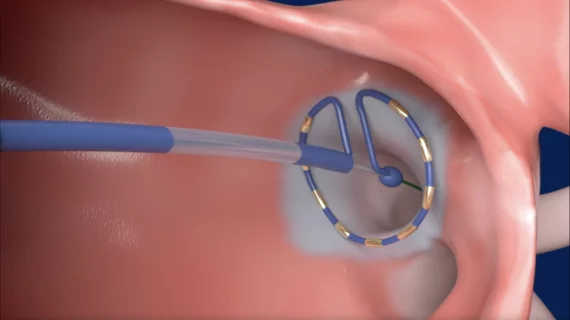
One cardiologist with experience using the newly approved system is Lucas V.A. Boersma, MD, PhD, a specialist with St. Antonius Hospital in the Netherlands. Boersma has been treating patients with PulseSelect and Medtronic’s 10F FlexCath Contour sheath.
“PulseSelect has the potential to redefine how atrial fibrillation is treated today. In our initial experience, we are already seeing the benefits of this technology over currently available single-shot therapies,” Boersma said in a statement. “The 10F sheath makes it easy to maneuver the catheter and is well visualized on any mapping system or fluoroscopy. The signal quality is excellent for post-ablation pulmonary vein validation, and full procedure times have been very short, just over 30 minutes, with no noticeable muscle contraction, which is beneficial for patient experience.”
“PulseSelect is clinically proven and offers the unique versatility of treating both paroxysmal and persistent AFib,” added Rebecca Seidel, president of Medtronic’s ablation solutions business. “The design lends itself well to providing therapy tailored to the patient’s anatomy. It’s a plug-and-play system that integrates easily with any workflow and handles exceptionally well. And our decade of research provides the peace of mind that comes with our commitment to safety.”
Because it uses electric pulses as opposed to extreme temperatures, many in the electrophysiology space believe PFA could eventually become the go-to treatment option over radiofrequency or cryoballoon ablation when AFib patients require care. With Medtronic and Boston Scientific now selling FDA-approved PFA systems, and other vendors such as Abbott working to catch up, this advanced technology continues to gain significant momentum.
Medtronic in Boston for AF Symposium 2024
AF Symposium 2024 in Boston is officially underway, and Medtronic is attending to display its latest heart rhythm technologies. The company has several presentations—some prerecorded and some live—planned for the three day event. Click here for the Medtronic’s full schedule.