Tricuspid Valve
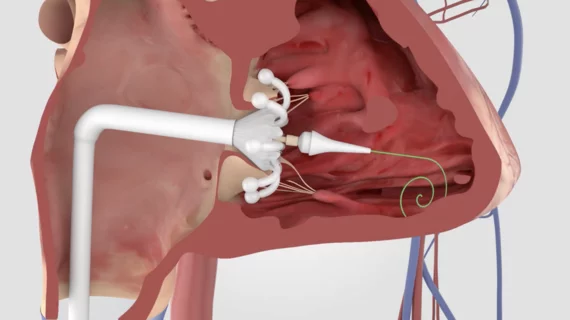
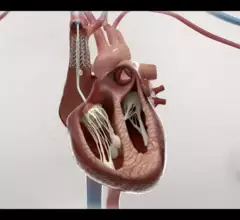
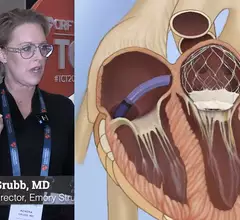
This channel is dedicated to news and trends in tricuspid valve interventions, surgical and transcatheter, to manage regurgitation and valve stenosis. The tricuspid valve is a rapidly growing area in structural heart interventions. Minimally invasive transcatheter tricuspid valve replacement (TTVR) and repair using transcatheter edge to edge repair (TEER) clips are expected to become a new standard of care in the next couple years, partly because open heart tricuspid surgery has historically has poor outcomes.
Displaying 25 - 32 of 55