Heart Rhythm
Hearts should have normal rhythm to their beats, but when these beats are out of synch, it causes inefficient pumping of blood. Irregular heart arrhythmias occur when the electrical signals that coordinate the heart's beats do not work properly. This can cause beats that are too fast (tachycardia), or too slow (bradycardia). Tachycardias include atrial fibrillation (AFib), supraventricular tachycardia, ventricular fibrillation, and ventricular tachycardia (VT). Bradycardias include sick sinus syndrome and conduction block. Electrophysiology arrhythmia treatments include medications, life style changes, and the EP lab interventions of catheter ablation, and implantable pacemakers or defibrillators.


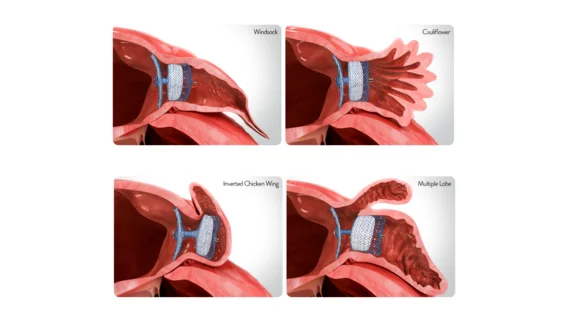



![Left atrial appendage occlusion (LAAO) with the Watchman FLX device from Boston Scientific is associated with positive outcomes and limited adverse events after one year, according to new findings published in Circulation: Cardiovascular Interventions.[1] Many prior Watchman FLX studies, including PINNACLE FLX, had focused on the device’s performance in a controlled setting. The study’s authors hoped to gain a better understanding of its real-world impact by reviewing registry data from more than 97,000 U.S](/sites/default/files/styles/240x220/public/2024-08/screenshot_2024-08-12_at_11.35.13_am.png.webp?itok=YxsSJPAl)
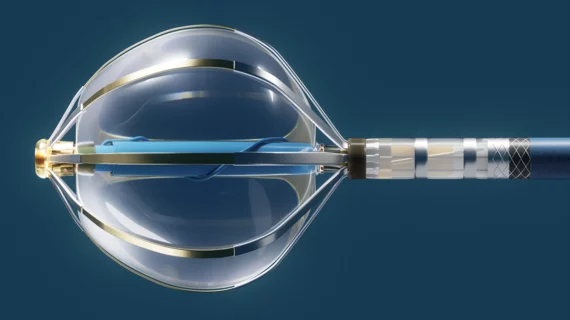
![Treating mitral regurgitation with transcatheter mitral edge-to-edge repair (TEER) using the MitraClip device is associated with a low risk of cerebrovascular accidents (CVAs) such as stroke and transient ischemic attack (TIA), according to new data published in The American Journal of Cardiology.[1]](/sites/default/files/styles/240x220/public/2024-08/small-tech-big-impact-mitraclip-960x430.jpg.webp?itok=mJO-jXba)
